The electrons go through an external circuit creating a flow of electricity. The stopping mechanism has to be controlled by a chemical reaction.
Sodium Batteries May Power Your New Electric Car Wired
The competition which involves multiple regional competitions and a final competition at the Annual Student conference increases.

. No obnoxious odor discharge is allowed. The AIChE Chem-E-Car competition is an annual event serving as an opportunity for chemical engineering undergraduates to utilize their knowledge and skills attained through engineering coursework and construct a small-scale vehicle powered by an unconventional source of chemical energy. The Chem-E Car team is split into five sub-teams.
We test additives structural changes and side reactions to optimize our power output and to get the power source competition ready. The Chemical Engineering Car Chem-E-Car is a shoe-box sized car that is powered by a chemical reaction. The Chem-E-Car Competition which involves multiple regional competitions and a final competition at the Annual Student conference increases.
A combination of wood material and rattan was used to build the reactor and car body. No commercial batteries are allowed as the power source. Some commonly used power sources include electrochemical batteries lead acid galvanic cell aluminum air or hydrogen fuel cell or thermoelectric generators.
AIChEs annual Chem-E-Car Competition engages college students in designing and constructing a car powered by a chemical energy source that will safely run a specific given distance and stop. Picture of Walt Prototype reaction vessels in back stopping mechanism in front. Electrochemical batteries such as lead acid galvanic cell hydrogen fuel cell or thermoelectric generators commonly used as power source for the Chem-E-Car Competition.
Our team is focused on providing power for the car through use of an aluminum air battery. The student-constructed vehicle is designed to. The power source is a Zn-Air battery.
This is quite a challenge because the distance and amount. The power source consists of 12 aluminum-air cells in series providing specific current and voltage needed to run the car. In this car design three reactors were used as the power source and were placed over the back wheels.
Power source can be reproducible on competition day and no liquid also odor discharge is allowed. Our car has become sleeker and our design has been much more improved through the learned lessons of the past. Never having been used before by the Berkeley Chem-E car team this.
Chem-E-Car is AIChEs annual competition engaging college students in designing and constructing a car powered by a chemical energy source that will safely carry a specified load over a given distance and stop. Never having been used before by the Berkeley Chem-E car team this project is focused on developing a battery that provides enough power to run the car and to get the power output as consistent as possible. We are an SDELC Design Team that designs a small car that is powered by a chemical reaction that produces electricity and is stopped by a timed reaction that can be modified by a limiting reactant.
The most popular stopping mechanism for the Chem-E-Car Competition is the Iodine Clock Reaction which involves mixing a form of iodine redox reagent and starch. The power source team researches tests and designs an energy source to power the car. Motor and power source are wired in series.
Fuel cella cell that converts fuel to electricity via an electrochemical reaction to power their carOne of the famous ones is the iodine clock reactionThis reaction works by using two clear solutions many variations that change color after a time delay the exact time can. The fuel cell is used to power two electric motors attached to the rear wheels that move the car forward. The car can run for a specific time and thus a specific distance given by the kinetics of a chemical reaction which produces foam with a known and controllable rate.
Each sub-team operates as an interdependent entity with project requirements set at the beginning of the design cycle. In a hydrogen fuel cell a catalyst at the anode separates hydrogen molecules into protons and electrons which take different paths to the cathode. Optimization of a Chem-E-Car New Jersey Governors School of Engineering and Technology 2014.
Efficient energy sources a car powered entirely by chemical reactions was built. The competition requires a payload of water up to 500mL to run a distance between 50 and 100. The power source of the car and brake system of the car is based on chemical reaction properties.
Steven Zheng Rueih Sheng Sophia Weng Andy Shin Abirami Murugappan Richard Sim Xinran Tian Matthew Helle Stephen Bagley Matthew Chang Justine Kang. UNNES has designed a Chem-E-Car prototype named Semarang Robotic Technology of UNNESs Chemical Cars or in short SMARTTRONS. Meanwhile the car will automatically stop by a colour change reaction.
Reactics Palapa is a chem-e-car that applies the concept of voltaic cell for its power source and uses the Chameleon reaction as its stopping mechanism. Since our first car in 2017 we have come a long way in developing professionally improving our car from the one in the video above. No brakes mechanical or electronic timing devices are allowed.
Chemical Engineering students of all levels contribute through their knowledge of chemical reactions physics circuits and thermodynamics. In accordance with AIChE regulations we have taken necessary steps to ensure Walt is safe to use in competition. A homemade zinc-air battery powers an electric motor driving the rear axle.
AIChEs annual Chem-E-Car Competition engages college students in designing and constructing a car powered by a chemical energy source that will safely carry a specified load over a given distance and stop. Oxygen is collected from. Power source for the propulsion of the car in Chem-E-Car Competition is a chemical reaction.
The Chem-E-Car means chemically energized car. IDEAS TILL NOW It does not matter how fast the car was but what matters is how accurately it covered a certain distance. No liquid discharge is allowed.
The competition which involves multiple regional competitions and a final competition at the Annual Student conference increases awareness of the chemical. The only energy source for the propulsion of the car is a chemical reaction. Power Source Stopping Mechanism Electrical Mechanical and Management.
The travel time is determined the produced foam volume.

Mcgill Chem E Car Design Team Home Facebook

Pure Electric Vehicle An Overview Sciencedirect Topics
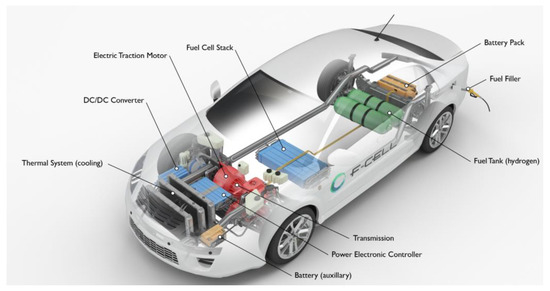
Applied Sciences Free Full Text Hydrogen Fuel Cell Vehicles Current Status And Future Prospect Html
0 Comments